Blood sugar control is a critical aspect of managing diabetes, a condition where the body struggles to regulate glucose levels in the blood.
Get semaglutide prescription online for weight loss
Get your online semaglutide prescription through Heally. Our platform connects you with licensed healthcare providers who can prescribe Wegovy (for weight loss) or Ozempic (for diabetes, if relevant) medications, delivering them straight to your door.
Weight Loss Program starting $199/Month

Weight loss results with semaglutide
Semaglutide helps many people lose weight. It’s the main ingredient in both Ozempic and Wegovy. Wegovy is approved for weight loss and uses a higher dose (2.4 mg weekly). Studies showed people using Wegovy lose up to 15% of their body weight in 68 weeks. Ozempic is FDA-approved for type 2 diabetes, but doctors sometimes prescribe it off-label for weight loss. It was studied at lower doses (up to 2 mg weekly). Studies showed loss between 4% to 9% of total body weight with Ozempic. Results take time. Many see changes after a few weeks and steady loss over months. Results may vary depending on adherence to treatment, diet, and exercise.
Not sure where to start with medicated weight loss
Our care team will explain all the medication options and connect you to a licensed provider so you can make the right decision for your weight loss goals.
Reviews from Real Patients
Patients have shared their experiences with Heally weight loss programs and semaglutide treatment. Many highlight noticeable changes in their health and habits, showing real results before and after semaglutide. Weight loss results vary and depend on diet, physical activity, and consistent use of the medication.





Get your semaglutide prescription online
Starting semaglutide treatment is simple with Heally. Our licensed doctors evaluate your health, discuss your weight loss goals, and determine if semaglutide is appropriate for you. Once approved, your medication may be shipped directly to your door. After prescription our doctor provides guidance on dosing, usage, and follow-up care – all without leaving your home.
Note: it's important to follow the prescribed dosage and consult with your doctor regularly to monitor your progress and address any concerns.
-
Schedule your appointment
Book a consultation with a licensed physician through the Heally’s telehealth platform. Our encrypted HIPAA-compliant platform keeps your medical information safe, secure, and confidential. -
Talk to a licensed doctor
Connect with a board-certified weight loss specialist who will review your health, discuss your weight management goals, and determine if this GLP-1 therapy is appropriate for you. The doctor may provide a prescription for the appropriate semaglutide medication (Wegovy, Ozempic or Rybelsus). -
Get your prescription and treatment plan
If approved, your doctor provides a personalized prescription along with guidance on dosage, usage, and monitoring to ensure safe, effective treatment. -
Support during your weight loss journey
You will get ongoing support during your semaglutide treatment, answering questions, monitoring progress, managing side effects and adjusting guidance as needed. With Heally, you have expert help every step of the way to stay on track and achieve your goals.
How semaglutide helps with weight loss
Semaglutide is the active ingredient in Wegovy and Ozempic. As a GLP-1 receptor agonist, Semaglutide mimics a natural hormone involved in appetite regulation. It works by increasing feelings of fullness, slowing stomach emptying, and reducing hunger signals to the brain. This helps you eat less and makes it easier to follow a lower-calorie diet. By supporting healthy eating habits, medication can lead to sustained weight loss when used alongside lifestyle changes and under medical supervision.
Semaglutide Medications for Weight Loss
Ozempic whose active ingredient is semaglutide, is FDA approved for type 2 diabetes, has shown to promote weight loss. Studies indicate that, on average, people using Ozempic for weight loss experience a 5-15% weight loss when combined with healthy lifestyle changes such as diet and exercise.

Self pay options starts at $200 depending on dose. No insurance needed. Wegovy, designed specifically for weight management comes as a once-weekly injection with a higher dosage than Ozempic, resulting in more substantial weight loss. In clinical trials, individuals using Wegovy for weight loss experienced an average weight loss of 15%.
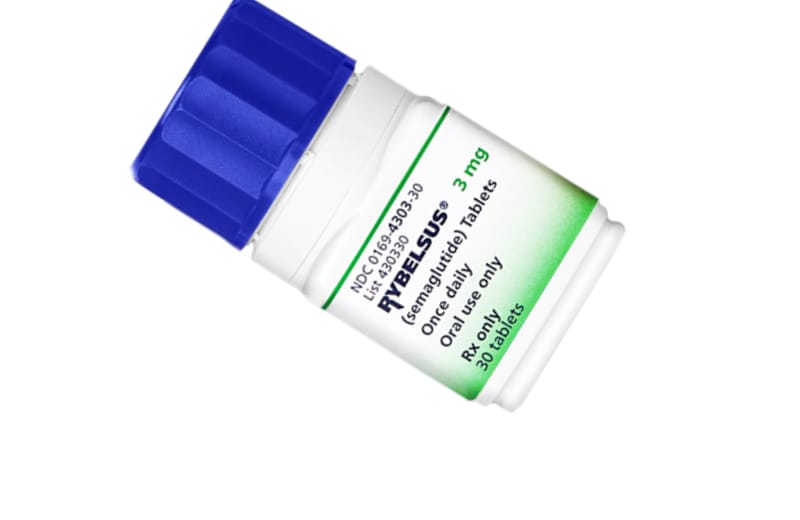
Rybelsus is the brand name for oral semaglutide, a pioneering medication designed for the management of type 2 diabetes. It is the first GLP-1 receptor agonist available in a tablet form, eliminating the need for injections. Rybelsus works by enhancing insulin secretion, suppressing appetite. Rybelsus is not FDA approved for weight loss.
Is semaglutide right for you?







Possible semaglutide side effects
-
Short-term side effects
These are most common when starting semaglutide or increasing your dose:
- Gastrointestinal effects: nausea, vomiting, diarrhea, constipation, and abdominal discomfort. These often improve as your body adjusts.
- Appetite changes: reduced hunger or occasional food aversions.
- Fatigue or dizziness: mild and temporary in many cases.
- Injection site reactions (for weekly injections): redness, itching, or swelling at the injection site.
-
Long-term or less common side effects
These are less frequent but important to be aware of:
- Pancreatitis: inflammation of the pancreas; persistent severe abdominal pain should be reported immediately.
- Gallbladder issues: such as gallstones or cholecystitis.
- Changes in blood sugar: especially for people with diabetes; hypoglycemia may occur if used with certain diabetes medications.
- Thyroid concerns: rare risk of thyroid tumors observed in animal studies; risk in humans is still being studied.
- Other effects: some patients report hair thinning or mild mood changes, but these are uncommon.
Most short-term side effects improve over time as your body adjusts. Regular follow-ups with your healthcare provider help monitor for serious or long-term effects. Side effects can often be managed by adjusting your dose or the timing of injections.
How to take semaglutide
-
For weekly semaglutide injections
- Administered under the skin (subcutaneous) in your thigh, abdomen, or upper arm.
- Start at a lower dose to reduce side effects, then gradually increase to the target dose as directed by your doctor.
- Take it on the same day each week for consistent effect.
- Rotate injection sites to avoid irritation.
-
For daily oral semaglutide
- Taken by mouth at the same time each day, typically on an empty stomach with water.
- Wait at least 30 minutes before eating, drinking, or taking other medications to ensure proper absorption.
Consistency is important — taking semaglutide as prescribed helps you get the best results. Combine your medication with a healthy diet and regular physical activity for optimal weight management.
Why choose Heally to get your prescription for semaglutide online?
Clinics using the Heally platform have been at the forefront of semaglutide and are knowledgeable in prescriptions such as Ozempic or Wegovy.

Lower semaglutide cost
Convenient 100% online experience
Seamless access to doctors
Expert guidance from licensed specialists
Cost of Semaglutide
Semaglutide treatments are now more affordable thanks to manufacturer coupons and discounts. Heally helps make therapy accessible by offering options like semaglutide coupons or a $100 program discount. These savings are designed to keep cost from standing in the way of your weight loss goals. Schedule a free consultation to learn about the latest semaglutide discount programs.
Semaglutide and insurance
Insurance coverage for semaglutide varies by provider. You can also use FSA, HSA, or Sezzle payment plans, and we offer discounts to help make treatment more affordable. Novo Nordisk currently provides eligible patients with first-month savings as low as $200, depending on the dose.FAQ: Semaglutide 101
Here are some answers to commonly asked questions related to the use of Semaglutide for weight loss.
-
What is Ozempic®?
This is a medication used to treat type 2 diabetes. It contains the active ingredient semaglutide, which belongs to the class of drugs called GLP-1 receptor agonists.
-
What is semaglutide compound in Ozempic®?
Semaglutide is the active ingredient in both Ozempic and Wegovy®. It is a synthetic version of a naturally occurring hormone called glucagon-like peptide-1 (GLP-1).
-
How does semaglutide work?
It works by mimicking the action of GLP-1, a hormone that helps regulate blood sugar levels. It stimulates the release of insulin, reduces the production of glucagon (a hormone that raises blood sugar), slows down gastric emptying, and promotes feelings of fullness.
-
What is Semaglutide’s dosing?
The dosing of semaglutide can vary depending on the individual's needs and the recommendation of their healthcare provider. It is typically administered once a week as a subcutaneous injection.
-
How to get Ozempic covered by insurance?
To determine coverage of Semaglutide medications by insurance, contact your insurance provider directly. Note that Ozempic (diabetes) may have different coverage than Wegovy (weight loss).
-
How many doses are in the Ozempic pen?
The Ozempic pen contains multiple doses. The specific number of doses per pen can vary, so it is important to check the packaging or consult the prescribing information for accurate details.
-
How does semaglutide help you lose weight?
It can help with weight loss by reducing appetite, increasing feelings of fullness, and slowing down gastric emptying. Weight loss may occur as a secondary effect of its primary action in managing blood sugar levels.
-
How long does it take to lose weight on Ozempic (semaglutide)?
Weight loss results on semaglutide can vary between individuals. Some people may start seeing weight loss within a few weeks, while others may take longer. Consistent use of it, along with a healthy diet and exercise, is important for achieving weight loss goals.
-
How much weight can I lose with Semaglutide for 1 month?
Weight loss on Semaglutide can vary. Clinical trials have shown weight loss is possible when used as prescribed, along with diet and exercise. Results depend on various factors such as adherence to treatment, individual metabolism, lifestyle changes, and overall health.
-
Is Semaglutide available on Heally?
Heally can connect you to doctors that can prescribe medication with semaglutide as the active ingredient, either Ozempic and Wegovy®. The medication is sold seperately directly at the pharmacy
-
How to get Semaglutide for weight loss?
To get Semaglutide for weight loss, it is necessary to consult with a healthcare provider. They will evaluate your medical history, assess your weight management goals, and determine if Semaglutide is suitable for you as part of a comprehensive weight loss plan.
-
How long can I stay on Semaglutide?
The duration of treatment can vary depending on individual circumstances. It is typically used as a long-term medication for managing type 2 diabetes or chronic weight management. The decision to continue Semaglutide should be made in consultation with a healthcare provider. They will assess your response to the medication, monitor your health, and determine the appropriate duration of treatment based on your specific needs and goals.
-
What are the side effects of Semaglutide?
Common side effects of semaglutide may include nausea, diarrhea, vomiting, abdominal pain, fatigue, headache, dizziness, weakness, irritability, and depression. Hair loss has been reported anecdotally by some patients taking GLP-1 medications, but it is not listed as a known side effect in FDA-approved labeling. It is important to report any concerning or persistent side effects to your healthcare provider.
-
Where to inject Semaglutide?
It is injected subcutaneously (under the skin) in the abdomen, thigh, or upper arm. It is recommended to rotate injection sites to reduce the risk of injection site reactions.
-
How long can Semaglutide be out of the fridge?
It should be stored in a refrigerator between 36°F (2°C) and 46°F (8°C). However, if necessary, unopened Ozempic pens can be stored at room temperature (below 86°F or 30°C) for a maximum of 56 days. After that, they should be discarded.
-
How to store Semaglutide?
It should be stored in the refrigerator between 36°F (2°C) and 46°F (8°C). It should be kept in its original packaging to protect it from light. Do not freeze Semaglutide or store it near the freezer compartment.
-
What foods to avoid while taking Semaglutide?
While taking Semaglutide, it is important to maintain a healthy diet. There are no specific foods to avoid, but it is advisable to follow a balanced meal plan that includes a variety of fruits, vegetables, whole grains, lean proteins, and healthy fats. Limit or avoid foods high in sugar, refined carbohydrates, and saturated fats.
-
Can I use Semaglutide and alcohol at the same time?
Moderate alcohol consumption is generally acceptable while using Semaglutide. However, alcohol can affect blood sugar levels and increase the risk of hypoglycemia (low blood sugar). It is important to drink responsibly and monitor blood sugar levels closely.
-
Can I use Ozempic while pregnant and breastfeeding?
Semaglutide is not recommended during pregnancy.. It is important to discuss pregnancy plans or confirm pregnancy before starting or continuing Semaglutide. For breastfeeding, it is not known if medicine passes into breast milk, so it is advisable to consult a healthcare provider to assess the potential risks and benefits.
-
Can people under 18 years of age be prescribed Semaglutide?
Ozempic is not approved for people under 18. Wegovy may be prescribed to patients 12 and older for weight management.
Weight loss terms and research
Lipid metabolism refers to the process by which the body handles fats, including their absorption, transportation, storage, and breakdown. It is a complex biological process essential for providing energy, maintaining cell structure, and producing hormones.
Semaglutide clinical trials refer to the research studies conducted to evaluate the effectiveness and safety of the medication semaglutide in treating conditions like type 2 diabetes and obesity.
Semaglutide Health And News

Ozempic for Weight Loss vs Other Medications
In today’s world, the prevalence of obesity is reaching alarming levels, making it one of the most significant public health concerns globally. In the United States…

Semaglutide Dosing Guidelines to Make Your Weight Loss Safe
Looking to shed excess pounds? The global epidemic of obesity has prompted an urgent search for safe and effective weight loss solutions…
FDA-Approved Drugs for Weight Loss: A Guide to the Best Options
With countless weight loss products flooding the market, it is crucial to separate the science-backed options from the fads.

With more than 30 years of physician practice in the Obstetrics and Gynecology specialty, Dr. Niles is recognized as a leading authority on the delivery of quality medical care. She has a well-established and respected history of worldwide medical collaboration, research, philanthropy, and distinguished leadership.
Important Disclaimers
Disclaimer: Individual results may vary. Semaglutide medications—Ozempic®, Wegovy®, and Rybelsus®—are FDA-approved for different indications. Wegovy® is approved for chronic weight management in adults who meet specific criteria, while Ozempic® and Rybelsus® are approved for type 2 diabetes. Weight loss outcomes depend on adherence to prescribed plans, diet, exercise, and individual response.
Ozempic®, Wegovy®, and Rybelsus® are registered trademarks of Novo Nordisk A/S. This website is not affiliated with, endorsed by, or sponsored by Novo Nordisk. Trademarks are used for informational and comparative purposes only.
Common side effects of semaglutide may include nausea, vomiting, diarrhea, abdominal pain, fatigue, dizziness, and constipation. Serious risks may include pancreatitis, gallbladder issues, kidney problems, and a potential increased risk of thyroid tumors. Semaglutide should not be used by individuals with a personal or family history of medullary thyroid carcinoma (MTC) or Multiple Endocrine Neoplasia syndrome type 2 (MEN 2). It is not recommended during pregnancy or while breastfeeding.
Inform your healthcare provider of all medications and supplements you are taking. Alcohol use may increase the risk of hypoglycemia. Semaglutide must be stored according to official guidelines—typically refrigerated, or at room temperature for a limited time if unopened. Refer to official packaging for full instructions.
Use of semaglutide for weight loss outside of FDA-approved indications is considered off-label. Any off-label use should be discussed with a licensed healthcare provider.
Program pricing, pharmacy costs, and eligibility for coupons or discounts may vary. Manufacturer savings programs and Heally promotions are subject to change and eligibility requirements.
The content on this page is for educational purposes only and does not constitute medical advice. Always consult a licensed healthcare provider before beginning, adjusting, or discontinuing any medication.
For full safety details and prescribing information, please refer to:
Data Last Updated 11/17/2025














