Get GLP-1 Weight Loss Medication Prescription
Book a consultation with a licensed healthcare provider through Heally to discuss whether GLP-1 medications, with active ingredients such as semaglutide or tirzepatide, or other medications like metformin, may be appropriate for you. Your provider will review your medical history and health goals before recommending any treatment. The treatment will include a personalized health plan to achieve your goals.
How to Connect with a Weight Loss Doctor Online Through Heally?
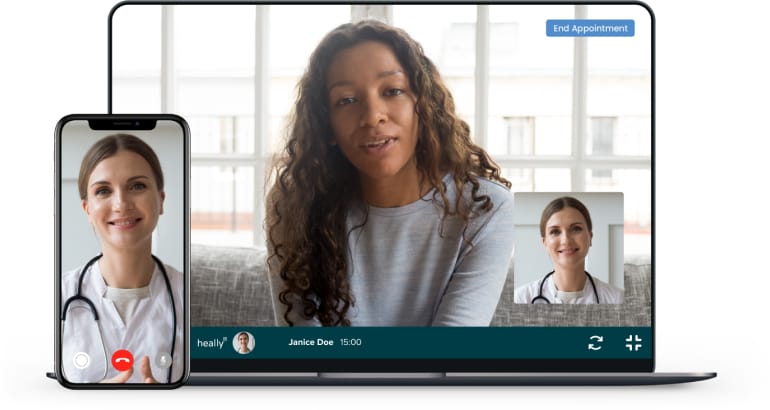
Start your weight management journey with Heally. Follow these steps to connect online with a licensed healthcare provider who can review your health history and discuss whether medical weight loss options, including GLP-1 medications, may be appropriate for you.
1: Sign Up with Heally
Begin by signing up on the Heally platform. Create your account to connect with licensed healthcare providers who offer online medical weight loss consultations. The sign-up process is quick and secure, and it is the first step in determining whether treatment may be right for you.
2: Schedule Your Appointment
Once registered, you can schedule an appointment for a weight loss consultation at a time that's convenient for you.
3: Weight Loss Doctor Consultation
During your online consultation, you'll be able to discuss your weight loss goals, medical history, and any concerns with your doctor. You can ask your doctor if GLP-1 weight loss medications may be right for you. Your doctor will assess your situation and, if appropriate, prescribe a medication for weight loss. If prescribed, it can be sent directly to your home.
Reviews about Weight Loss Drugs
GLP-1 Medications for Weight Loss
Price starts at $299 from Eli Lilly Direct and shipped directly to your home. No insurance necessary. Zepbound (tirzepatide) is an FDA approved weight loss medication that targets both GLP-1 and GIP receptors. In contrast to Semaglutide, which solely focuses on the GLP-1 receptor, Zepbound’s innovative approach not only aids in significant weight loss but also enhances overall metabolic health more effectively.
Ozempic whose active ingredient is semaglutide, is FDA approved for type 2 diabetes, has shown to promote weight loss. Studies indicate that, on average, people using Ozempic for weight loss experience a 5-15% weight loss when combined with healthy lifestyle changes such as diet and exercise.

Wegovy, designed specifically for weight management comes as a once-weekly injection with a higher dosage than Ozempic, resulting in more substantial weight loss. In clinical trials, individuals using Wegovy for weight loss experienced an average weight loss of 15%.
Rybelsus is the brand name for oral semaglutide, a pioneering medication designed for the management of type 2 diabetes. It is the first GLP-1 receptor agonist available in a tablet form, eliminating the need for injections. Rybelsus works by enhancing insulin secretion, suppressing appetite. Rybelsus is not FDA approved for weight loss.
Mounjaro (tirzepatide) is a medication that acts on both GLP-1 and GIP receptors, offering a dual mechanism to enhance weight loss and improve glucose control, making it distinct from other semaglutide treatments like ozempic for weight loss.
Liraglutide is a GLP-1 receptor agonist medication used for weight management and type 2 diabetes treatment. Available under brand names Victoza (for diabetes) and Saxenda (for weight loss), it helps regulate blood sugar and reduce appetite. Compounded liraglutide offers a customized approach to weight loss, working by mimicking a natural hormone that targets areas of the brain controlling appetite and food intake.

What weight loss medication is good for you?







Loss Medication?
Why Choose Heally to Get Weight Loss Products Online?
Heally stands out as a premier telehealth platform, expertly connecting patients with specialized weight loss doctors, who can prescribe GLP-1 medications to reduce weight. Our platform is designed to streamline your journey towards achieving your health and weight loss goals, providing an accessible and effective way to obtain weight loss medication.
Access to the Latest Medications
Doctor Network
Customized Weight Loss Plans
100% Online Process
Book Your Consultation with Heally
Weight Loss Drug Mechanism of Action
Glucagon-like peptide-1 (GLP-1) is an incretin hormone that plays a crucial role in glucose metabolism and weight regulation. For weight loss, GLP-1 works by slowing gastric emptying, which helps increase satiety and reduce appetite, leading to lower caloric intake. Additionally, GLP-1 enhances insulin secretion in response to meals and suppresses glucagon release, which helps maintain stable blood glucose levels. These combined effects contribute to weight loss by promoting a feeling of fullness and reducing food intake, while also improving metabolic functions. GLP-1 receptor agonists, such as Semaglutide and Tirzepatide, mimic the action of natural GLP-1, making them effective treatments for obesity and type 2 diabetes management.
Using GLP-1 receptor agonists for weight loss shows impressive results. Clinical trials demonstrate that medications like Semaglutide and Tirzepatide lead to substantial weight reduction in patients with obesity or those who are overweight. On average, individuals treated with GLP-1 agonists experience a weight loss of around 10-15% of their initial body weight over a period of 6 to 12 months.The effectiveness and timeline for seeing results can vary based on the individual's baseline weight, adherence to the medication regimen, and lifestyle factors.

GLP-1 Weight Loss Drug Side Effects
While GLP-1 receptor agonists may support weight loss, they can also cause side effects. Common side effects include gastrointestinal issues such as nausea, vomiting, diarrhea, and constipation. These are most likely when starting treatment and may lessen over time. Some patients experience decreased appetite, which can lead to reduced food intake. Less commonly, GLP-1 agonists may cause pancreatitis, gallbladder problems, or kidney issues. Hypoglycemia is uncommon but can occur, particularly when used with other diabetes medications. Other possible side effects include injection site reactions, headache, and dizziness. Not all patients will experience these effects, and individual responses vary.
To help reduce side effects, healthcare professionals often recommend starting with a low dose and gradually increasing it. Staying hydrated, eating a balanced diet, and regular monitoring may also help manage discomfort. Patients should report any adverse effects to their doctor, as treatment adjustments may be needed. For detailed information about a specific drug’s side effects, please see its dedicated page.
FAQ
-
When to start using weight loss products?
Weight loss products should be considered when lifestyle changes such as diet and exercise have not been successful, and you are seeking additional support for weight management. It's important to consult with a healthcare provider to determine the right time to begin medication.
-
How do weight loss drugs work?
Weight loss drugs work through various mechanisms, such as suppressing appetite, increasing metabolism, or reducing the absorption of dietary fat, to help reduce body weight. The exact mechanism depends on the medication's active ingredients.
-
What are the most common side effects of weight loss medications?
Common side effects include nausea, constipation, headache, dry mouth, and sometimes more serious risks like increased heart rate or blood pressure changes. Discuss potential side effects with your healthcare provider.
-
What is GLP-1?
GLP-1 (Glucagon-like peptide-1) is a naturally occurring hormone involved in glucose metabolism and appetite regulation. It helps control blood sugar levels by enhancing insulin secretion and inhibiting glucagon release. GLP-1 also slows gastric emptying and increases feelings of fullness, which can aid in weight loss.
-
What are GLP-1 weight loss medications, and how do they work?
GLP-1 weight loss medications, such as Semaglutide and Tirzepatide, mimic the action of the natural hormone GLP-1. They help regulate appetite by slowing gastric emptying, increasing feelings of fullness, and reducing food intake, which leads to effective weight loss. They also enhance insulin secretion and stabilize blood glucose levels.
-
Who can benefit from GLP-1 weight loss medications?
GLP-1 weight loss medications are suitable for individuals with obesity or those who are overweight and have not achieved desired results with diet and exercise alone. They are also beneficial for people with type 2 diabetes who need to manage both weight and blood glucose levels.
-
How to get weight loss medications?
Weight loss medications can be prescribed after a consultation with a healthcare provider, who will evaluate your health status and weight loss needs. Heally offers a platform to connect with weight loss doctors online for this purpose.
-
What is the most effective weight loss product?
The effectiveness of weight loss products varies by individual and their specific health conditions. Medications like Semaglutide, Metformin, and Tirzepatide have shown promising results in clinical studies.
-
When will I see the results after using weight loss medications?
Results can vary widely, some individuals may start to see weight loss within the first few weeks of medication use, with more significant results over months of consistent use alongside lifestyle changes.
-
What are the common side effects of GLP-1 weight loss medications?
Common side effects include gastrointestinal issues such as nausea, vomiting, diarrhea, and constipation. Some patients may also experience decreased appetite, headaches, dizziness, and injection site reactions. These side effects are usually mild and tend to improve over time.
-
How much weight can I expect to lose with GLP-1 medications?
Clinical studies have shown that individuals using GLP-1 medications like Semaglutide and Tirzepatide can lose approximately 10-20% of their initial body weight over a period of 6 to 12 months. Results can vary based on individual factors and adherence to treatment.
-
Who are the best weight loss doctors to get consultation?
The best weight loss doctors are those who specialize in obesity medicine or endocrinology and have experience managing patients with weight loss medications. Heally connects you with qualified doctors for GLP-1 weight loss consultations.
-
How much does weight loss consultation cost?
Free consultations are available for any questions you may have.
-
Why choose Heally to connect with a weight loss doctor?
Heally offers a convenient and secure way to consult with experienced weight loss doctors from anywhere, providing access to the latest weight loss medications and personalized care plans.
-
Are weight loss medications safe?
When prescribed by a healthcare provider and used according to guidance, weight loss medications can be a safe part of a comprehensive weight management plan. It's crucial to discuss any health conditions and potential drug interactions with your doctor.

With more than 30 years of physician practice in the Obstetrics and Gynecology specialty, Dr. Niles prides herself on the delivery of quality medical care. She has a well-established and respected history of worldwide medical collaboration, research, philanthropy, and distinguished leadership.
Data Last Updated 12/04/2025