As a dual-agonist, tirzepatide-based medications targets two key hormones involved in regulating blood sugar and appetite. Clinical studies have shown tirzepatide to be associated with significant weight management outcomes. As a dual-agonist, this medic$ation targets two key hormones involved in regulating blood sugar and appetite. The FDA has approved tirzepatide under two brand names for different indications: Mounjaro for type 2 diabetes management and Zepbound for chronic weight management in adults with obesity or overweight with weight-related medical conditions. While tirzepatide can be highly effective, following the prescribed tirzepatide dosing schedule is crucial for ensuring both safety and efficacy.
This information is for educational purposes only and should not replace professional medical advice. All dosing decisions must be made exclusively by qualified healthcare providers based on individual medical assessment. Never adjust medication doses without medical supervision.
Tirzepatide Dosing for Weight Loss
Tirzepatide for weight loss is FDA-approved, but only under the brand name Zepbound, which received approval in November 2023 for chronic weight management among adults with obesity or overweight with related health conditions. This article refers to tirzepatide in the context of Zepbound—while Mounjaro contains the same active molecule, its FDA approval is specifically for improving glycemic control in adults with type 2 diabetes, not for weight loss.
When prescribed for chronic weight loss management, tirzepatide dosing typically starts at a lower dose to minimize potential side effects and is gradually increased. This approach helps patients adjust to the medication and allows healthcare providers to monitor tolerance and watch out for adverse effects.
The dosing guidelines can help to optimize treatment outcomes, so it is important that you listen to your healthcare provider and follow the prescribed tirzepatide dose schedule.
Tirzepatide Dosing Schedule
Tirzepatide medication is provided in pre-filled, single-dose injector pens. These pens are available at a specific tirzepatide dose. This can be:
- 2.5 mg/0.5 mL
- 5 mg/0.5 mL
- 7.5 mg/0.5 mL
- 10 mg/0.5 mL
- 12.5 mg/0.5 mL
- 15 mg/0.5 mL
You’ll notice this is different from other GLP-1 medications like semaglutide. Unlike tirzepatide, which can go up to 15 mg, semaglutide maxes out at 2.4 mg doses. This is not because one is more effective than the other. Each medication works differently and targets specific patient needs based on their unique pharmacological profiles.
Though each individual’s tirzepatide dosing schedule may vary, treatment typically begins with the tirzepatide starting dose and gradually increases from there. Following the initial dose, patients will gradually increase their tirzepatide dose every 4 weeks to minimize side effects and allow the body time to adjust to the medication.
Healthcare providers determine individual dosing schedules based on patient-specific factors, medical history, and treatment response.
What is the starting dose for Tirzepatide?
The tirzepatide dosing schedule for weight loss typically starts with an initial dose of 2.5 mg once weekly.
After four weeks, the dose can be increased to 5 mg once weekly, depending on the patient’s response and tolerance. Dose adjustments are generally made in 2.5 mg increments every four weeks.
What is the max dose of Tirzepatide?
The tirzepatide max dose is 15 mg/0.5 mL. However, each individual’s max dose will vary. The maximum maintenance dose for each patient will be determined by their healthcare provider.
Your healthcare provider will consider your side effects and progress when determining your maintenance dose.
Tirzepatide Dosing Chart
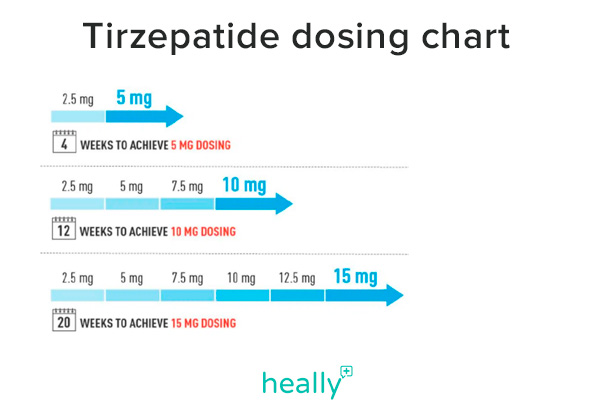
The tirzepatide dosing schedule is ultimately set by your healthcare provider, but Eli Lilly (the manufacturer of both Mounjaro and Zepbound) has designed a tirzepatide dosing chart that most providers will follow.
The medication is intended to increase at 2.5 mg every 4+ weeks, depending on how your body adjusts.
Tirzepatide Starting Dose |
Dosage Increase |
Tirzepatide Dose Schedule |
New Tirzepatide Dose |
| 2.5 mg/0.5 mL | +2.5 mg | 4+ weeks | 5 mg/0.5 mL |
| 5 mg/0.5 mL | +2.5 mg | 4+ weeks | 7.5 mg/0.5 mL |
| 7.5 mg/0.5 mL | +2.5 mg | 4+ weeks | 10 mg/0.5 mL |
| 10 mg/0.5 mL | +2.5 mg | 4+ weeks | 12.5 mg/0.5 mL |
| 12.5 mg/0.5 mL | +2.5 mg | 4+ weeks | 15 mg/0.5 mL |
| 15 mg/0.5 mL | N/A | N/A | N/A |
How to Adjust your Tirzepatide Dose
When it comes to maximizing the benefits of tirzepatide, knowing when and how to adjust your doses is key. The recommended schedule, which typically spans at least four weeks for each dose level, is designed to accommodate the varying tolerance levels and adjustment periods among different individuals.
However, it is important to note that you should not adjust your tirzepatide dose on your own.
Your tirzepatide dosing schedule should be determined by your healthcare provider, not you. This helps ensure that your treatment is both effective and safe.
Here are key steps for adjusting your tirzepatide dose:
- Start Low: Begin with the lowest available dose (2.5 mg once weekly). This initial dose helps your body get used to the medication.
- Monitor Response: Pay close attention to how your body responds. Note any side effects and improvements in weight loss and overall health.
- Consult Your Healthcare Provider: After the initial four weeks, discuss your progress with your healthcare provider. They will assess your tolerance and decide if it’s appropriate to increase the dose.
- Gradual Increases: If tolerated well, your provider may recommend increasing the dose to 5 mg once weekly. This process continues in increments, typically every four weeks, with potential increases to 7.5 mg, 10 mg, 12.5 mg, and up to a maximum of 15 mg once weekly.
- Personalize the Schedule: The pace at which you increase your dose can be adjusted based on your individual needs and responses. Some people may need more time at each dose level to minimize side effects and maximize benefits.
- Ongoing Communication: Maintain regular communication with your healthcare provider throughout your treatment. This ensures any necessary adjustments can be made promptly and safely.
Follow proper dosing of tirzepatide
Following the proper tirzepatide dosing schedule is essential for achieving the best weight loss results while ensuring your safety. As everyone’s body responds differently, it’s important to work closely with your healthcare provider to tailor the dosing regimen to your specific needs.
Heally connects patients with licensed medical providers who evaluate medical history, provide personalized recommendations, and monitor progress over time. Through virtual consultations, Heally makes it easy to explore whether a tirzepatide-based treatment plan—or an alternative—is right for you. Our team offers continued follow-up and support, ensuring your care is safe, data-driven, and tailored to your needs.
A healthcare professional can provide personalized guidance, monitor your progress, and make necessary adjustments to your dosage. Schedule a consultation with Heally today to discuss your tirzepatide dosing schedule.
Sources
- New England Journal of Medicine: Tirzepatide Once Weekly for the Treatment of Obesity
- Eli Lilly: Your weekly Zepbound routine
- DOM: Efficacy and tolerability of tirzepatide, a dual glucose-dependent insulinotropic peptide and glucagon-like peptide-1 receptor agonist in patients with type 2 diabetes: A 12-week, randomized, double-blind, placebo-controlled study to evaluate different dose-escalation regimens
- Eli Lilly: Mounjaro ® (tirzepatide) injection
- Drugs.com: Tirzepatide
Medical Disclaimer: This article explains the differences between compounded tirzepatide vs regular tirzepatide for educational purposes only. It does not provide medical advice. Compounded medications do not have FDA approval and may pose different risks than FDA-approved options. Consult a qualified healthcare provider before starting any medication.
Important Current Status Update: As of March 19, 2025, the FDA no longer allows most pharmacies to compound tirzepatide. The FDA ended enforcement discretion after resolving the drug shortage. Now, patients must use FDA-approved medications such as Mounjaro® and Zepbound®.
Important Medical Information and Disclaimers
MEDICAL DISCLAIMER
This information is for educational purposes only and is not intended as medical advice, diagnosis, or treatment recommendation. Tirzepatide is available through two FDA-approved prescription medications: Mounjaro® (tirzepatide) injection is approved for improving blood sugar control in adults with type 2 diabetes mellitus, and Zepbound® (tirzepatide) injection is approved for chronic weight management in adults with obesity or overweight with weight-related medical problems. While both medications contain the same active ingredient (tirzepatide), they are approved for different therapeutic indications and may have different dosing regimens. Always consult with a qualified healthcare provider before starting, stopping, or changing any treatment.
INDIVIDUAL RESULTS VARY
Results from tirzepatide treatment vary significantly between individuals. Clinical trial results may not reflect real-world outcomes for all patients. Factors that may influence treatment results include adherence to prescribed dosing, implementation of lifestyle modifications (diet and exercise), individual metabolic responses, underlying health conditions, concurrent medications, and genetic factors. No treatment outcome can be guaranteed.
IMPORTANT SAFETY INFORMATION
Common Side Effects may include nausea, vomiting, diarrhea, decreased appetite, constipation, stomach pain, heartburn, belching, gas, and injection site reactions. These effects are often temporary and may decrease over time.
Always discuss your complete medical history, current medications, and any concerns with your healthcare provider before starting treatment. Regular monitoring and follow-up appointments are essential during treatment.
FDA ADVERSE EVENT REPORTING: You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088. Healthcare providers and patients can also report adverse events to the manufacturer.